COVID-19 is an acute severe respiratory syndrome caused by a novel coronavirus 2 (SARS-CoV-2), which appeared in 2019. As of June 1, 2021, it has infected more than 170 million people[1]. Common initial symptoms and signs include cough, fever, fatigue, headache, muscle aches, and diarrhea[2]. Symptoms typically appear about one week after infection. Some COVID-19 patients develop severe symptoms, and respiratory distress is one of the most common symptoms that can progress to respiratory failure, often accompanied by hypoxemia. Severe COVID-19 infections can lead to respiratory failure, life-threatening pneumonia, multi-organ failure, and even death[3].
What are the prevention and treatment methods for COVID-19?
Effective COVID-19 vaccines are crucial for preventing further morbidity and mortality. Vaccines targeting COVID-19 may protect against infection, disease, or transmission, providing protection against severe cases and death, contributing to disease control[4]. Currently, many countries are involved in vaccine development, and each vaccine development strategy has distinct advantages and disadvantages[5]. It is especially important to vaccinate high-risk populations, including frontline healthcare workers, individuals over 60 years of age, and those with chronic conditions (especially diabetes and hypertension)[6].
What COVID-19 vaccines are currently available?
Coronaviruses are spherical RNA viruses with a particle size of about 100 nm. Currently, there are three subgroups and more than 16 types of coronaviruses discovered[7]. Two-thirds of the SARS-CoV-2 genome encodes the replicase, while one-third encodes the protease. They use their self-synthesized replicase and protease to produce four structural proteins: spike protein (S), envelope protein (E), membrane glycoprotein (M), and nucleocapsid protein (N). The constant evolution of the novel coronavirus enables it to spread rapidly in populations[8].
Many studies have shown that designing vaccines targeting the spike protein (S) is the most effective approach. The S protein is responsible for attaching the virus to the host cell’s surface receptor, most likely the angiotensin-converting enzyme-2 (ACE2) in type II alveolar cells, making antibodies against the S protein crucial for interfering with the virus and neutralizing infections caused by it[9]. Consequently, many COVID-19 vaccine development platforms are designed and developed specifically for the S protein[8].
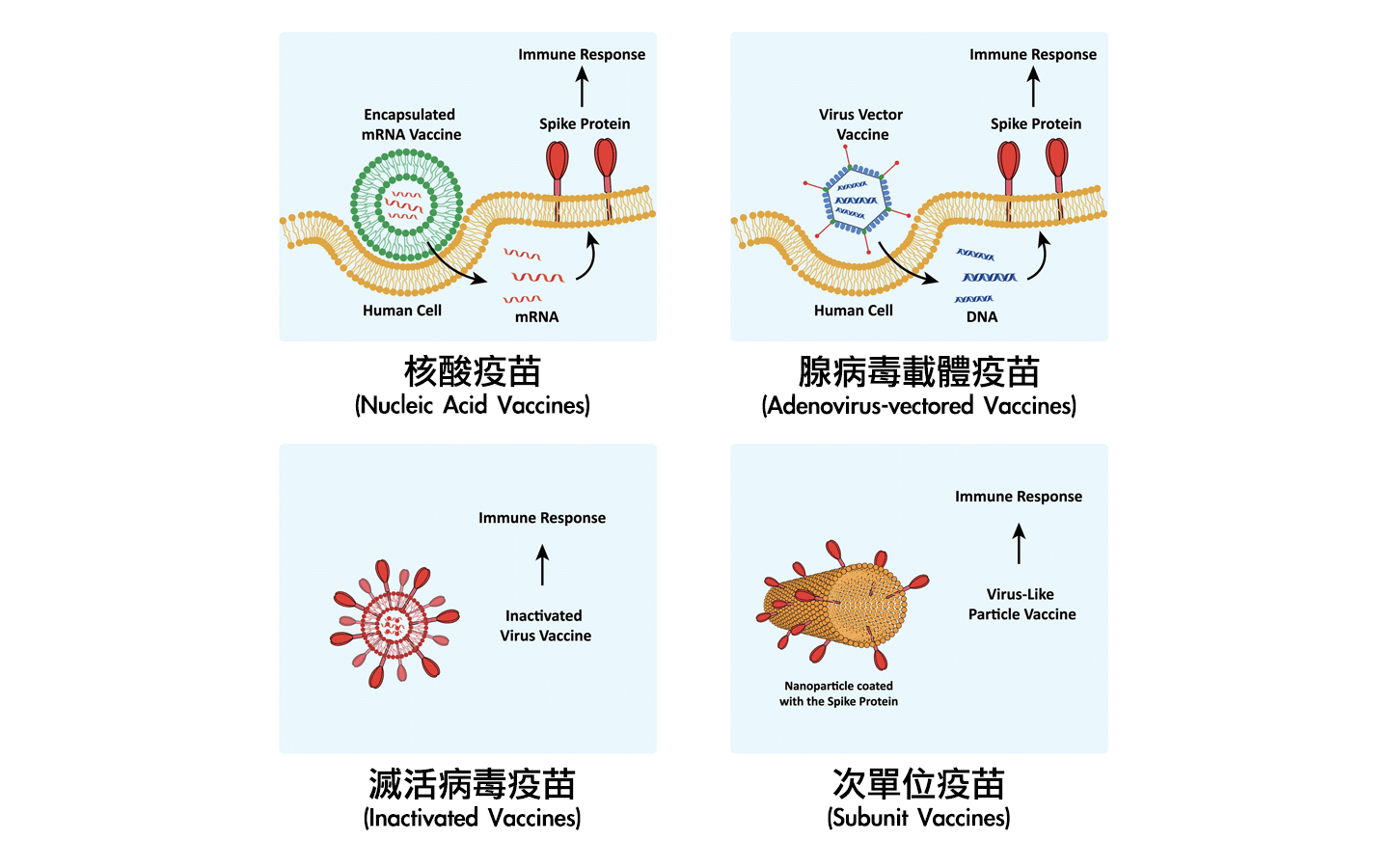
Currently, there are various types of COVID-19 vaccines, including inactivated vaccines, adenovirus-vectored vaccines, subunit vaccines, and nucleic acid vaccines[5].
What are the differences between COVID-19 vaccines?
-
Inactivated Vaccines
Inactivated or non-active whole-virus vaccines are commonly used in virus vaccine development. They essentially weaken the virus’s activity using different techniques. Since live or inactivated whole viruses are injected into the body, they primarily induce protective antibodies against the angiotensin-converting enzyme-2 (ACE2) protein on the virus’s surface. These types of virus vaccines usually require extensive testing to confirm their safety.
Furthermore, because live or inactivated whole SARS-CoV-2 virus vaccines have been associated with increased infectivity after immunization, this poses a serious problem for COVID-19 vaccines[5] [8]. These types of vaccines are mainly developed by the Wuhan Institute of Biological Products and China National Pharmaceutical Group (Sinopharm) and are inactivated SARS-CoV-2 virus vaccines[10].
- Adenovirus-vectored Vaccines
The principle of this type of vaccine involves inserting the gene that can produce the primary antigen of the novel coronavirus, the spike protein, into a vector using genetic recombination technology. This vector cannot replicate but can enter human cells directly. By doing so, it utilizes the body’s cells to produce the spike protein, thereby stimulating the body’s immune response.The most commonly used viral vector is the adenovirus vector, with the primary advantage being its ability to induce both humoral and cellular immunity[8]. Examples of vaccines in this category include Ad26.CoV2.S developed by Janssen (Johnson & Johnson) using a chimpanzee adenovirus as the vector and ChAdOx1 nCov-19 developed through collaboration between the University of Oxford and AstraZeneca, which uses a human adenovirus type 26 as the vector[10].
- Subunit Vaccines
Highly purified recombinant proteins from various pathogens are the most common development technique in vaccine research[8]. Subunit vaccines for SARS coronaviruses are primarily based on eliciting an immune response against the S-spike protein to prevent its binding to the host’s angiotensin-converting enzyme-2 (ACE2) receptor. These vaccines consist of the receptor-binding domain (RBD) of the S-spike protein of SARS-CoV. Through genetic recombination, the gene that can produce the antigenic spike protein is inserted into the cell’s DNA. After substantial cultivation of genetically modified cells, the purified spike protein is obtained, creating subunit protein vaccines.
Furthermore, subunit vaccines induce neutralizing antibodies without causing Th2-type immune pathology. An advantage of RBD-type vaccines is their ability to minimize host immune enhancement[5] [11]. Examples of vaccines in this category include NVX-CoV2373 developed by Novavax (USA), MVC-COV1901 developed by Medigen Vaccine Biologics (Taiwan), and UB-612 developed by United Biomedical (Taiwan), among others[10]. - Nucleic Acid Vaccines
Several major biotechnology companies have advanced COVID-19 nucleic acid vaccine platforms. Inovio Pharmaceuticals is developing a DNA vaccine, while other companies like Moderna Therapeutics (USA) and Curevac are opting for RNA vaccine platforms[5]. In general, RNA vaccines have several advantages over DNA vaccines and can directly inject mRNA into host cells, where translation occurs in the cytoplasm.These vaccines have been successfully tested with different antigen vaccines in many species, including mice, non-human primates, and humans[12]. Examples of vaccines in this category include mRNA-1273 developed by Moderna (USA) and BNT-162b2, developed through collaboration between Pfizer (USA) and BioNTech (Germany), among others[10].
Comparison of Side Effects of Different COVID-19 Vaccines
In this comparison, we’ll directly examine the side effects of COVID-19 vaccines commonly available in Taiwan or internationally[13] [14].
| Pfizer/BioNTech (BNT) | AstraZeneca (AZ) | Moderna | Janssen (Johnson & Johnson) | Medigen Vaccine Biologics | |
| Vaccine Type | mRNA | Adenovirus Vector | mRNA | Adenovirus Vector | Subunit Protein |
| Dosage | 0.3 mL | 0.5 mL | 0.5 mL | 0.5 mL | (To Be Announced) |
| Administration Route | Injection into Muscle | Injection into Muscle | Injection into Muscle | Injection into Muscle | Injection into Muscle |
| Age for Administration | 16 years and older | 18 years and older | 18 years and older | 18 years and older | 20 years and older |
| Number of Doses | 2 doses | 2 doses | 2 doses | 1 dose | 2 doses |
| Efficacy after Two Doses | 95% | 81% | 94% | 67% | (To Be Announced) |
| Interval Between Doses | 4 weeks | 10-12 weeks | 4 weeks | – | 4 weeks |
| Common Side Effects | Headache, Fatigue, Redness at Injection Site, Nausea, Chills | Headache, Fever, Fatigue, Muscle Pain, Joint Pain, Nausea, Swelling at Injection Site, Vomiting, Thrombocytopenia, Chills, Diarrhea, Rash | Headache, Fever, Fatigue, Muscle Pain, Joint Pain, Nausea, Swelling at Injection Site, Vomiting, Enlarged Lymph Nodes, Erythema | Pain at Injection Site, Fever, Itching, Swelling, Bruising | Pain at Injection Site, Erythema, Swelling, Muscle Soreness, Fatigue, Fever, Diarrhea |
| Less Common Side Effects | Myocarditis, Enlarged Lymph Nodes, Facial Paralysis | Thrombosis, Dizziness, Drowsiness, Sweating, Itching, Rash, Enlarged Lymph Nodes | Myocarditis, Itching at Injection Site, Facial Swelling, Facial Nerve Paralysis | Rare Coagulation Disorders, Thrombosis |
Conclusion
There are various types of COVID-19 vaccines available, and there is a risk of allergic reactions with each vaccine. Therefore, individuals can discuss with their healthcare provider to choose the vaccine that is more suitable for their individual health conditions. After receiving the vaccine, it’s important to monitor any changes in your body within 30 minutes and up to 28 days after vaccination. If you experience any adverse reactions, please seek medical attention promptly, and inform the healthcare provider that you have received a COVID-19 vaccine. At present, the global COVID-19 pandemic continues, and getting vaccinated remains a global trend in combating the disease. Of course, maintaining personal hygiene, including frequent handwashing and mask-wearing, is also crucial.
Read Next



